Articles
Sections
Calculating the nutrients loss in food processing
July 25, 2021
Keywords:
nutrients loss
food processing
raw
cooked
boiled
2- Is drinking sea water good for your health?
It depends.
Does "sea water" means:
- pure, unfiltered, or filtered sea water?
- surface sea water or deep sea water?
- undiluted sea water, or diluted (for example: isotonic or hypertonic)
- desalinated sea water?
- desalinated and remineralized seawater?
Does 'drinking' means:
- drink exclusively "sea water" or alternate with fresh water?
- drink "sea water" permanently or for certain periods of time?
Drinking permanently and exclusively pure sea water, unfiltered or filtered, from the surface or from 200 meters deep, undiluted, is not good for health.
" Seawater contains salt. When humans drink seawater, their cells are thus taking in water and salt. While humans can safely ingest small amounts of salt, the salt content in seawater is much higher than what can be processed by the human body. Additionally, when we consume salt as part of our daily diets, we also drink liquids, which help to dilute the salt and keep it at a healthy level. Living cells do depend on sodium chloride (salt) to maintain the body’s chemical balances and reactions; however, too much sodium can be deadly. Human kidneys can only make urine that is less salty than salt water. Therefore, to get rid of all the excess salt taken in by drinking seawater, you have to urinate more water than you drank. Eventually, you die of dehydration even as you become thirstier." [0]
3- Experiments on the beneficial effects of Deep Sea Water (DSW)
This article [1] (2016) reviews the proven effects of deep sea seawater (DSW) administered orally or tested in vitro. Most of the subjects in the experiments are mice and rabbits; The experiments made on people give us a reference of what seawater is being used for testing in human.
Treatmen of skin problems:
(31) human, oral, DSW 1000 hardness, 500 ml/day, 6 months
(32) human, oral, DSW 1000 hardness, 500 ml/day, 3 weeks
Effects of deep sea water on Hepatic Protection:
(19) human, in vitro, HepG2 cells, DSW 200, 400, 600, 800, and 1000 hardness, 24 hr
Effects of deep sea water on fatigue:
(35) human, oral, DSW 710 hardness, fatiguing exercise conducted for 4 hr at 30°C
Effects of deep sea water on antibacterial activity:
(42) human, oral, DSW hardness of 100, 250, 500, and 1000
(36) human, in vitro, DSW hardness of 1200 and 2400
(42) human, oral, DSW hardness: 1000, 1 L/daily, 10 days
Effects of deep sea water in the liver and kidney status:
(4) human, oral, DSW 1410 hardness, supplemented 1050 mL daily, 6 weeks
In all cases, this is deep sea water (DSW), not surface water (SSW). DSW is usually desalinated and / or concentrated, and finally diluted, basically due to its high magnesium (Mg) content which has to fit with the Limit Mg RDA (Recommended Dietary Allowance).
In most cases it is prepared in different concentrations of salts; in each case the hardness of the sea water is reported.
* "Deep sea water (DSW) commonly refers to a body of seawater that is pumped up from a depth of over 200 m. It is usually associated with the following characteristics: low temperature, high purity, and being rich with nutrients, namely, beneficial elements, which include magnesium, calcium, potassium, chromium, selenium, zinc, and vanadium. Less photosynthesis of plant planktons, consumption of nutrients, and organic decomposition have caused lots of nutrients to remain there. Due to this, DSW has potential to become a good source for health. Research has proven that DSW can help overcome health problems especially related to lifestyle-associated diseases such as cardiovascular disease, diabetes, obesity, cancer, and skin problems. This paper reviews the potential health benefits of DSW by referring to the findings from previous researches." [1]
Hardness Formula:
The same article explains how Hardness is calculated:
Hardness = Mg (mg/l) * 4.1 + Ca (mg/l) * 2.5
Some of the experiments use different proportions of Ca and Mg.
The hardness formula can give the same result for different ratios of Ca: Mg.
Some conclusions in the article:
"Through the impressive findings of DSW benefits to health, it is suggested that its utilization should be promoted widely."
"The effort to put DSW as a water source that is beneficial for health should be enhanced."
So, drinking diluted deep sea water periodically (in addition to fresh water), can be good for health.
4- Deep Sea Water versus Surface Sea Water
Sea Water Composition:
[ Table 1 ]
Element |
SSW |
DSW |
Na |
10800 |
7240 |
K |
392 |
10400 |
Ca |
411 |
39 |
Mg |
1290 |
96100 |
Sr |
8.1 |
0.17 |
B |
4.45 |
320 |
Fe |
0.003 |
0.25 |
Li |
0.17 |
11.7 |
Cu |
0.0009 |
0.22 |
Co |
0.0004 |
0.26 |
Mo |
0.01 |
0.62 |
Ni |
0.0066 |
0.11 |
Cr |
0.0002 |
0.087 |
Rb |
0.12 |
1.2 |
Si |
2.9 |
0.5 |
V |
0.002 |
1.2 |
F |
13 |
21.8 |
Br |
67.3 |
5400 |
I |
0.064 |
5.5 |
* Data source: see [1]
If we look at Table 1, we can see that SSW and DSW have a surprisingly different compositions; But the difference related to Mg is amazing. In DSW Mg concentration is 96110 mg/l while in SSW it is 1290 mg/l. Also the concentrations are very different for Na, Ca, K, B and Br the most abundant nutrients in SSW and DSW.
We can also observe that, for most nutrients, the values are much higher in DSW than in SSW, except in Na, Ca, Sr and Si..
In this article [2] the difference between deep sea water and surface sea water in the preventive effect of atherosclerosis was investigated. DSW and SSW solutions were prepared by desalination, concentrating and diluting in distilled water. The authors conclude that DSW showed a positive effect on rabbit cells but no effect was observed with SSW.
Having SSW and DSW so different compositions, it is clear that experiments on DSW give no information about the results that the same experiments would give if SSW was used instead of DSW.
5- What about drinking Surface Sea Water (SSW)?
Deep Sea Water is not what we are interested in; we want to know about drinking 'seawater', and now we have seen that it is called Surface Sea Water (SSW), the one we can take directly from the sea we bath in, the one that is unlimited, free and accessible.
6- Sodium tolerable upper limits (UL)
At this article [4] we can read about current RDA and UL for Sodium (Na):
"DIETARY REFERENCE INTAKES FOR SODIUM: There remains insufficient evidence to establish sodium DRIs for adequacy as EARs and RDAs. Furthermore, there is insufficient evidence to establish a toxicological risk level from high sodium intake, separate from chronic disease risk. As such, no sodium UL is established."
"There is sufficient evidence to characterize the relationship between sodium intake and risk of chronic disease. The CDRR is established using evidence of the beneficial effect of reducing sodium intake on cardiovascular disease risk, hyper-tension risk, systolic blood pressure, and diastolic blood pres-sure. Reduction of sodium intakes above the sodium CDRR is expected to reduce chronic disease risk within the apparently healthy population"
At this article [2] we can read about Sodium (Na) toxicity:
"An acute toxicity from excess sodium intake with the possibility of fatal outcome has been reported in relation to the ingestion of huge amounts of sodium, such as 0.5–1 g of salt/kg body weight. In certain pathologic conditions (e.g., heart failure, decompensated liver cirrhosis, and renal failure), sodium intake to levels routinely present in our diet (≥10 g/d) may lead to a dangerous increase in ECF volume.
However, even under normal conditions, the intake of high amounts of sodium tends to favor, especially in predisposed individuals, an increase of ECF volume and BP. The Intersalt study (2) showed that the higher the habitual consumption of sodium in a given population, the stronger the average BP increase with age and the prevalence of hypertension."
The same article explains:
" the Institute of medicine defined an 'Adequate Intake' AI of 2300 mg of Sodium (or 5.75 g of salt) per day for all adult individuals. The recommendations by the U.K. Food Standard Agency and the Sodium Working Group of the Minister of Health for Canada are in line with those by the IOM.
Recent WHO guideline has set ≤2000 mg of sodium (5 g of salt) per day as a target for the population stating that this level is fully compatible with the prophylaxis of thyroid diseases caused by iodine deficiency, which can be prevented by more extensive use of iodized salt. (5)."
7- Sodium Chronic Disease Risk Reduction Intake (CDRR)
As we have shown above: " insufficient evidence to establish a toxicological risk level from high sodium intake, separate from chronic disease risk"
Therefore, based on the body of evidence found in this study [4] (2019) of National Academies of Science, Engineering and Medicine, a Chronic Disease Risk Reduction intake (CDRR) was defined in order to recommend an upper limit for Sodium.
Conclusions of this large study:
Table2 - Chronic Disease Risk Reduction Intake (CDRR) by Age Group
Nutrient | Population Group | Recommendation |
|---|---|---|
Sodium | Children, 1–3 years | Reduce intakes if above 1,200 mg/day |
Children, 4–8 years | Reduce intakes if above 1,500 mg/day |
|
Adolescents, 9–13 years | Reduce intakes if above 1,800 mg/day |
|
Adolescents, 14–18 years | Reduce intakes if above 2,300 mg/day |
|
Adults, ≥ 19 years | Reduce intakes if above 2,300 mg/day |
8- How much sodium is there in 1 liter of surface isotonic seawater (SSW)?
* Isotonicity: a solution is isotonic when its effective osmole concentration is the same as that of another solution. In biology, the solutions on either side of a cell membrane are isotonic if the concentration of solutes outside the cell is equal to the concentration of solutes inside the cell. In this case the cell neither swells nor shrinks because there is no concentration gradient to induce the diffusion of large amounts of water across the cell membrane. Water molecules freely diffuse through the plasma membrane in both directions, and as the rate of water diffusion is the same in each direction, the cell will neither gain nor lose water [5].
The osmolarity of normal saline, 9 grams NaCl dissolved in water to a total volume of one liter, is a close approximation to the osmolarity of NaCl in blood (about 290 mOsm/L). Thus, normal saline is almost isotonic to blood plasma [5].
Taking normal saline as a reference:
'To convert Hypertonic (surface) seawater (35‰) to Isotonic seawater (9‰), the dilution rate is: 30% pure ocean water to 70% diluting water - by volume. Or one takes ordinary seawater and dilutes it with Spring Water by the ratio of 2:4.66 which means: 2 L of pure ocean water is mixed with 4.66 L of diluting water to produce 6.66 L of isotonic ocean water.'[6]
So if we take 300 ml to prepare 1 liter of seawater isotonic solution, we have:
300ml(SSW)*10800(mg Sodium)/1000ml(SSW)= 3240 mg Sodium (or 8.170 gr of NaCl)
This is 40% more than the CDRR of Sodium (2300 mg of sodium / day for ≥ 14 years individuals). This, not counting the Sodium ingested from food.
Here we have a conflict.
If we know approximately what our daily sodium intake is and want it to fit with sodium CDRR to reduce the risk of
chronic diseases, but at the same time we want to take advantage of SSW nutrients, but we do not want to buy either
SSW or DSW, we can reduce sodium intake in our diet to be able to drink daily maybe 750 ml of isotonic SSW.
This is feasible.
Try our calculator to find which is your current sodium intake
9- Reasons to drink isotonic surface seawater (SSW)
Well, this is the detailed composition of the surface water of the sea, with all the elements of the periodic table, as potential nutrients:
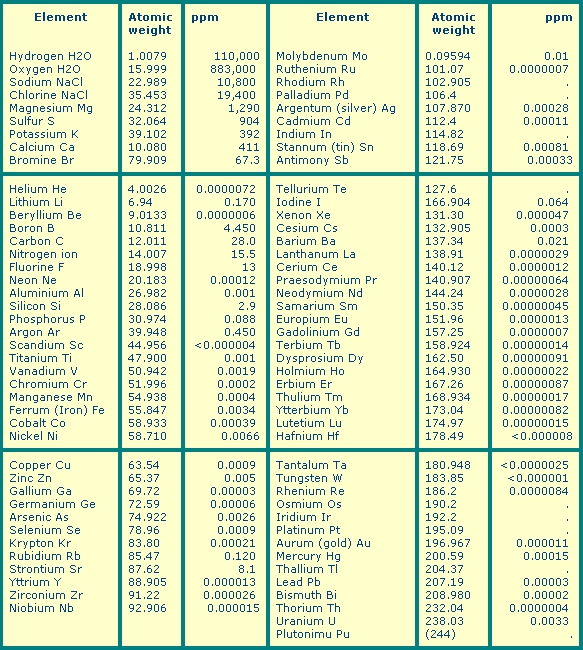
Note: ppm= parts per million = mg/litre = 0.001g/kg.
source: Karl K Turekian: Oceans. 1968. Prentice-Hall
Data from www.oceanplasma.org [6]
And this is the similarity between Surface Sea Water composition and extra-cellular liquid, blood plasma and intra-cellular liquid:
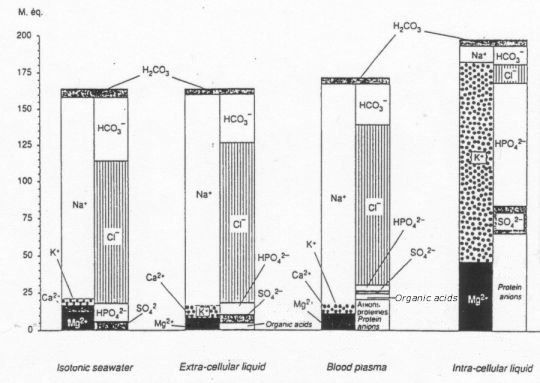
Note: Comparison of electrolyte compositions in isotonic ocean water, extra-cellular liquid, blood plasma and intra-cellular liquid. The height of each column represents the total electrolyte concentration.
Data from www.oceanplasma.org [7]
And this is how this article ends for us, by citing part of a study from the same last source [7]:
"(...) In addition, the results of treatments using correctly formulated ocean water preparations demonstrate the amazing therapeutic efficacy of such ocean water. How can this be explained? What are the links between ocean water and the vital internal environment? As a final analysis, what is the influence of ocean water on the ionic and mineral balance of the organism?
In this study, we discuss the research and therapeutic applications of Ocean Plasma as published by René Quinton. His findings can be briefly summarized as follows:
1. The precursors of the first human cells were undoubtedly unicellular organisms and thus they were the ancestors of human beings.
2. These unicellular cells lived in ocean water. Their needs in terms of trace elements and mineral salts were met by solely by the Ocean Water. Also, the buffering capability of the ocean environment provided the acid-base (acid-alkaline) balance.
3. Following a great many zoological observations that will not be discussed here, Quinton maintained that, in order to ensure complete cellular development, the human organism preserved as its internal environment a medium similar to that of the oceans at the time when cellular life began.
4. Quinton demonstrated that isotonic ocean water, that is the marine liquid medium reduced to the concentration of an organism's internal environment, has remained the preferred internal environment of human and animal cells.
5. He postulated that from the mineral point of view, human and Ocean Plasma are environments of the same nature. In other words, "there is physical and physiological identity between ocean water and the internal environment of the organism". Not only do they exhibit very similar mineral compositions but the particular form, organization and synergy of trace elements and mineral salts that make up the saline matrix of ocean water closely resemble those of the body's internal environment constituents.
6. The different assumptions made by Rene Quinton involved a whole series of facts borne out by zoological observations and experiments. Then Quinton surveyed the possible medical applications of his findings.
7. Together with a medical team, for more than 25 years, Quinton developed the famous "marine method" based on "Quinton's Plasma" or "Ocean Plasma", as an injectible isotonic solution of ocean water.
The works of physicians Jarricot, Robert Simon, Lachèze, Macé and Quinton, all rely on the principle of regenerating the depleted internal environment by means of purified ocean water. This results in a balanced and complete composition, so as to allow the patient to systematically reconstruct the internal terrain, the cells, and thus establish homeostasis.
The work of Quinton and his collaborators dealt with various types of cutaneous disorders, neuro-vegetative asthenias, anorexia, acute cachexia, infant diarrhea, deep dehydrations, gastro-enteritis, pulmonary tuberculosis, cholera, and typhus.
The exceptional results which were obtained from the discoveries and work of these pioneers should now be supplemented by new research. Their writings and the listed clinical cases demonstrate the great therapeutic attractiveness of Quinton's research findings and the efficacy of his method."
10- References
[0] https://oceanservice.noaa.gov/ facts/drinksw.html
[1] Mohd Nani, Samihah Zura et al. “Potential Health Benefits of Deep Sea Water: A Review.”
Evidence-based complementary and alternative medicine : eCAM vol. 2016 (2016): 6520475. doi:10.1155/2016/6520475
[2] Difference between Deep Seawater and Surface Seawater in the Preventive Effect of Atherosclerosis
Mitsuhiko MIYAMURA, Saburo YOSHIOKA, Atsuhide HAMADA, Daisuke TAKUMA, Junko YOKOTA,
Masahiko KUSUNOSE, Shojiro KYOTANI, Hirohisa KAWAKITA, Kazuhiro ODANI, Yasuyuki TsUTSUI and Yutaka NISHIOKA,
a: Department of Pharmacy, Kochi Medical School Hospital; Kohasu, Oko-cho, Nankoku, Kochi 783–8505, Japan
b: Kochi Prefectural Deep Seawater Laboratory; 7156 Aza Maruyama, Murotomisaki-cho, Muroto, Kochi 781–7101, Japan
c: Muroto Marinefood Corporation; 3507–5 Murotomisaki-cho, Muroto, Kochi 780–8123, Japan: and d Odanikokufun
Corporation; 939–4 Takasu, Kochi, Kochi 780–8123, Japan.
Received July 13, 2004; accepted September 9, 2004; published online September 10, 2004
[3] Strazzullo P, Leclercq C. Sodium. Adv Nutr. 2014 Mar 1;5(2):188-90. doi: 10.3945/an.113.005215. PMID: 24618759; PMCID: PMC3951800.
[4] Review of the Dietary Reference Intakes for Sodium and Potassium (2019)
National Academies of Science, Engineering and Medicine
https://www.nationalacademies.org/ our-work/ review-of-the-dietary- reference-intakes-for -sodium-and-potassium
[5] Sperelakis, Nicholas (2011). Cell Physiology Source Book: Essentials of Membrane Biophysics. Academic Press. p. 288
[6] https://oceanplasma.org/ documents/chemistry.html, SEA SALT CONCENTRATIONS
[7] https://oceanplasma.org/ documents/passbecsoulier-e.html